
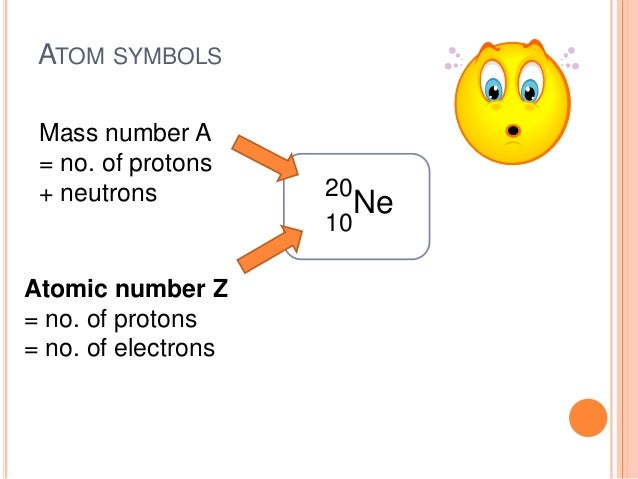
In this notation, the atomic number is not included. The atomic number A: equal to the number of protons (placed as a left subscript) 3. Symbol-mass format for the above atom would be written as Cr-52. The symbol X: the usual element symbol 2. For an example of this notation, look to the chromium atom shown below:Īnother way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. A property closely related to an atom’s mass number is its atomic mass. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number. Similarly, the isotope whose atomic weight is 24.986 amu has a mass number of 25, 13 neutrons, and 25 Mg as a symbol. number of an atom is defined as the number of protons present in its nucleus, the atomic number of the given element is 35. Together, the number of protons and the number of neutrons determine an element’s mass number: mass number protons + neutrons. The isotope whose atomic weight is 23.985 u has a mass number of 24 (protons and neutrons), so 24 - 12 protons gives 12 neutrons. The "A" value is written as a superscript while the "Z" value is written as a subscript. There are 12 protons in all magnesium isotopes. Both the atomic number and mass are written to the left of the chemical symbol. For example, Carbon had an atomic weight of 12.00 in 1902 but today it is 12.

Since 1899 the IUPAC Commission on Isotopic Abundances and Atomic Weights has been evaluating atomic weights and abundances. The truth is that atomic weights have changed as a function of time. The composition of any atom can be illustrated with a shorthand notation called A/Z format. Atomic weights found within a periodic table one might think are constant.


 0 kommentar(er)
0 kommentar(er)
